EXPERT INSIGHTS
DESIGN OF BIO/MEDICAL PRODUCTS
DESIGN OF BIO/MEDICAL PRODUCTS

BRIDGING SCIENCE & SAFETY
BRIDGING SCIENCE & SAFETY
Getting to the Bottom of Bio/Medical Product Failures
Getting to the Bottom of Bio/Medical Product Failures
In the intricate world of biomedical product design, safety and compliance with regulatory standards are paramount. Forensic engineering plays a crucial role in ensuring the integrity and efficacy of medical devices, human cell and tissue products (HCT/Ps), biologics, stem cells, and exosomes. When a medical product fails, our experts at Compass Consulting Engineers delve into the complexities of regulatory compliance in product realization to identify the root cause of the product failure or subsequent injury.
In the intricate world of biomedical product design, safety and compliance with regulatory standards are paramount. Forensic engineering plays a crucial role in ensuring the integrity and efficacy of medical devices, human cell and tissue products (HCT/Ps), biologics, stem cells, and exosomes. When a medical product fails, our experts at Compass Consulting Engineers delve into the complexities of regulatory compliance in product realization to identify the root cause of the product failure or subsequent injury.

Ensuring Regulatory Compliance
Ensuring Regulatory Compliance
By scrutinizing the design, manufacturing processes, and intended use of these products, our bio/medical experts can help medical device manufacturers navigate the complex FDA and international regulatory landscapes.
Does your team need an outside expert? Our staff provides consulting in Regulatory Affairs and Quality Assurance (RA/QA) for product development, manufacturing, failure analysis, QMS gap assessments, CAPA, and complaint handling.
By scrutinizing the design, manufacturing processes, and intended use of these products, our bio/medical experts can help medical device manufacturers navigate the complex FDA and international regulatory landscapes.
Does your team need an outside expert? Our staff provides consulting in Regulatory Affairs and Quality Assurance (RA/QA) for product development, manufacturing, failure analysis, QMS gap assessments, CAPA, and complaint handling.
Comprehensive Analysis
Comprehensive Analysis
Our engineers and scientists conduct comprehensive analyses of medical device and biomedical product facilities and Device Master Records (DMR) to assess their adequacy.
Our team can thoroughly examine records for medical devices and biomedical products to ensure regulatory compliance and detect potential reasons for adverse events in the field. There is always a paper trail. Let us review your Device History File, SOPs, risk management files, FMEA, and other areas of documentation required by the FDA to determine if a product’s failure occurred long before it was used in a clinical setting.
MITIGATING ROADWAY HAZARDS
Understanding Guardrails & Edge Drop
Compass Consulting Engineers specializes in evaluating the effectiveness of guardrails and assessing edge drop scenarios to enhance roadway safety. Our analysis includes thorough examination of roadside hazards and their impact on accident patterns, enabling us to identify design flaws and implement corrective measures. By understanding the dynamics of the road environment, we provide valuable insights that mitigate risks for motorists and prevent accidents.


Collision Mitigation
Guardrails serve as protective barriers along roads and highways. They shield vehicles from hazards such as steep drop-offs, trees, or other obstacles.
During a collision, guardrails absorb impact forces, preventing vehicles from careening off the road and potentially saving lives.
Energy Channeling
When a vehicle collides with a guardrail, it channels energy away from the vehicle. The guardrail transforms kinetic energy into deformation energy, minimizing injury severity.
Properly designed and placed guardrails play a crucial role in redirecting collision forces and reducing the risk of more severe accidents.

How OUR EXPERTS Can Help
HOW OUR EXPERTS CAN HELP
Navigating Regulatory Challenges: Our team of forensic engineers and scientists specializes in guiding clients through the intricate landscape of FDA regulations and industry standards. We offer expert assistance to those affected by failures of medical devices or biologics.
Analysis and Investigation: With a focus on comprehensive analysis and testing, we delve deep into the intricacies of medical device failures. Our forensic engineers meticulously examine the root causes of device malfunctions, providing valuable insights for you to use in your strategy.
Navigating Regulatory Challenges:
Our team of forensic engineers specializes in guiding clients through the intricate landscape of FDA regulations and industry standards. We offer expert assistance to individuals facing litigation due to medical device complications, ensuring that your case meets all necessary regulatory requirements.
Analysis and Investigation: With a focus on comprehensive analysis and testing, we delve deep into the intricacies of medical device failures. Our forensic engineers meticulously examine the root causes of device malfunctions, providing valuable insights to support your litigation strategy.



Understanding FMEA
Understanding FMEA
In the intricate world of biomedical product design and manufacturing, ensuring safety and efficacy is paramount. FMEA stands for Failure Modes and Effects Analysis, a systematic methodology used to identify and prioritize potential failure modes within a process, system, or product, and assess the impact of these failures. This proactive process requires a thorough, preemptive analysis of the design and manufacturing processes of biomedical products-including medical devices, implants, and biologics—including a corresponding risk and likelihood assessment. By methodically evaluating potential failure modes, such as material defects, component malfunctions, or human error, product developers can identify vulnerabilities that may compromise product safety and efficacy and put controls in place to mitigate those risks.
In the intricate world of biomedical product design and manufacturing, ensuring safety and efficacy is paramount. FMEA stands for Failure Modes and Effects Analysis, a systematic methodology used to identify and prioritize potential failure modes within a process, system, or product, and assess the impact of these failures. This proactive process requires a thorough, preemptive analysis of the design and manufacturing processes of biomedical products-including medical devices, implants, and biologics—including a corresponding risk and likelihood assessment. By methodically evaluating potential failure modes, such as material defects, component malfunctions, or human error, product developers can identify vulnerabilities that may compromise product safety and efficacy and put controls in place to mitigate those risks.
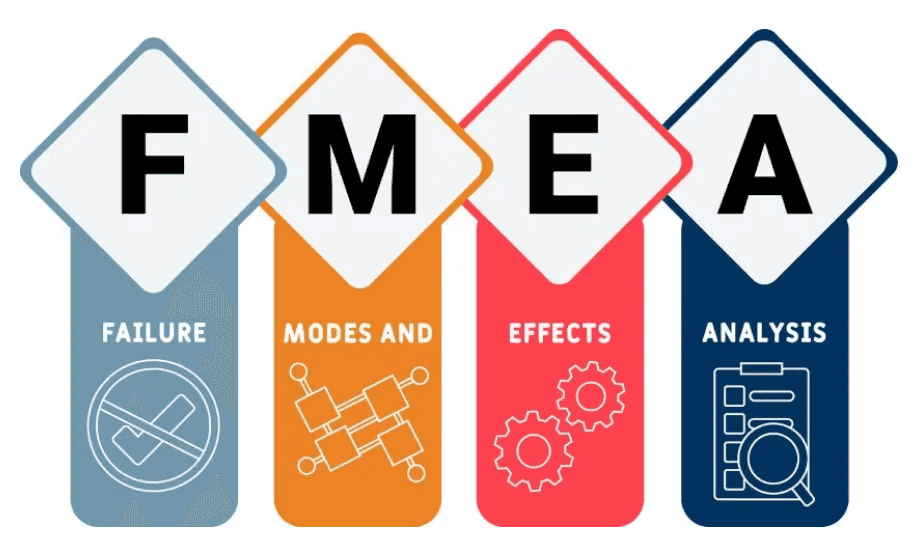


Unraveling Failure Modes
Unraveling Failure Modes
Detailed Failure Analysis: Compass Consulting Engineers conducts reviews of companies’ FMEA files, as well as conducts retroactive FMEA analyses to deconstruct the sequence of events leading to the product failure. By scrutinizing design specifications, manufacturing processes, and user requirements, we can help identify failure modes and their underlying causes.
Identifying System Weaknesses: Through FMEA, we pinpoint vulnerabilities within the product's design, materials, or manufacturing processes that contributed to a failure. This deep dive enables us to develop a comprehensive understanding of the systemic issues at play.
MITIGATING ROADWAY HAZARDS
Understanding Guardrails & Edge Drop
Compass Consulting Engineers specializes in evaluating the effectiveness of guardrails and assessing edge drop scenarios to enhance roadway safety. Our analysis includes thorough examination of roadside hazards and their impact on accident patterns, enabling us to identify design flaws and implement corrective measures. By understanding the dynamics of the road environment, we provide valuable insights that mitigate risks for motorists and prevent accidents.

Collision Mitigation
Guardrails serve as protective barriers along roads and highways. They shield vehicles from hazards such as steep drop-offs, trees, or other obstacles.
During a collision, guardrails absorb impact forces, preventing vehicles from careening off the road and potentially saving lives.
Energy Channeling
When a vehicle collides with a guardrail, it channels energy away from the vehicle. The guardrail transforms kinetic energy into deformation energy, minimizing injury severity.
Properly designed and placed guardrails play a crucial role in redirecting collision forces and reducing the risk of more severe accidents.
RELATED EXPERT INSIGHTS
LET’S WORK TOGETHER
We’re looking forward to hearing how we can help.
Call our office at 720-458-9190 or click below.
TELL US ABOUT YOUR CASE


LET’S WORK TOGETHER
We’re looking forward to hearing how we can help.
Call our office at 720-458-9190 or click below.
TELL US ABOUT YOUR CASE


LET’S WORK TOGETHER
We’re looking forward to hearing how we can help.
Call our office at 720-458-9190 or click below.
TELL US ABOUT YOUR CASE
Corporate Headquarters
Compass Consulting Engineers PC
10875 Dover St., # 900 Westminster, CO 80021
Pacific Northwest Region
Compass Consulting Engineers PC
13036 SE Kent-Kangley Rd, Suite 7, Kent, WA 98030
Canadian Region
Compass Consulting Engineers Inc.
1975 McCallum Road, Unit 115 #1029, Abbotsford, BC V2S 3N3
Corporate Headquarters
Compass Consulting Engineers PC
10875 Dover St., # 900 Westminster, CO 80021
Pacific Northwest Region
Compass Consulting Engineers PC
13036 SE Kent-Kangley Rd, Suite 7, Kent, WA 98030
Canadian Region
Compass Consulting Engineers Inc.
1975 McCallum Road, Unit 115 #1029, Abbotsford, BC V2S 3N3
Corporate Headquarters
Compass Consulting Engineers PC
10875 Dover St., # 900 Westminster, CO 80021
Pacific Northwest Region
Compass Consulting Engineers PC
13036 SE Kent-Kangley Rd, Suite 7, Kent, WA 98030
Canadian Region
Compass Consulting Engineers Inc.
1975 McCallum Road, Unit 115 #1029, Abbotsford, BC V2S 3N3